
What is most remarkable to consider is that a hemoglobin molecule is comprised of two alpha and two beta chains that each consist of about 150 amino acids. Specifically, valine in the β chain substitutes the amino acid glutamic. In sickle cell anemia, the hemoglobin β chain (a small portion of which we show in Figure) has a single amino acid substitution, causing a change in protein structure and function. A change in nucleotide sequence of the gene’s coding region may lead to adding a different amino acid to the growing polypeptide chain, causing a change in protein structure and function. The gene encoding the protein ultimately determines the unique sequence for every protein. Note that all disulfide bonds are the same length, but we have drawn them different sizes for clarity. Two disulfide bonds connect the A and B chains together, and a third helps the A chain fold into the correct shape. Two sulfhydryl groups can react in the presence of oxygen to form a disulfide (S-S) bond. The amino acid cysteine (cys) has a sulfhydryl (SH) group as a side chain. In each chain, three-letter abbreviations that represent the amino acids' names in the order they are present indicate primary structure. Bovine serum insulin is a protein hormone comprised of two peptide chains, A (21 amino acids long) and B (30 amino acids long). The amino acid sequences in the A and B chains are unique to insulin.
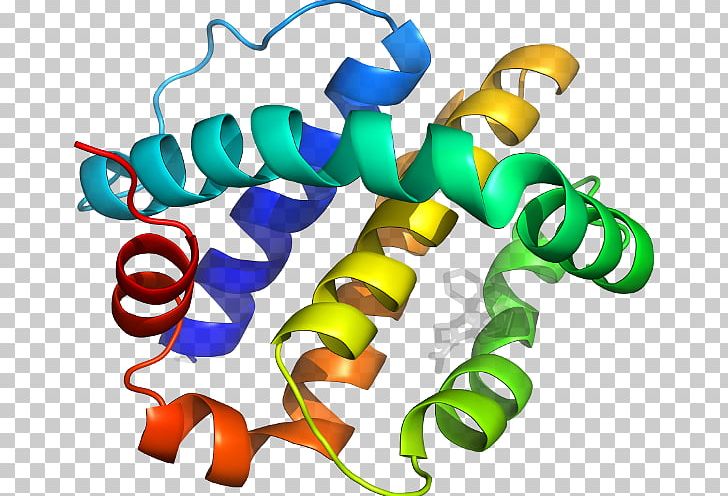
The N terminal amino acid of the A chain is glycine whereas, the C terminal amino acid is asparagine ( Figure). For example, the pancreatic hormone insulin has two polypeptide chains, A and B, and they are linked together by disulfide bonds. Primary StructureĪmino acids' unique sequence in a polypeptide chain is its primary structure. To understand how the protein gets its final shape or conformation, we need to understand the four levels of protein structure: primary, secondary, tertiary, and quaternary.
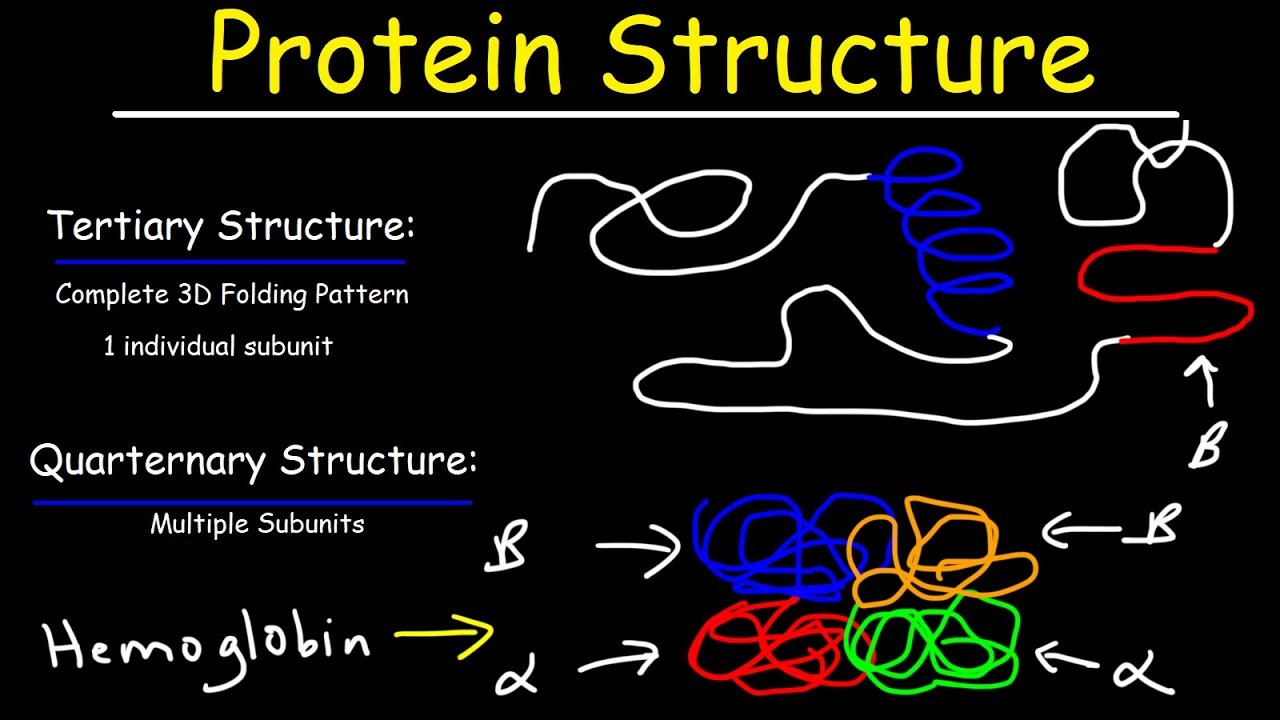
If this active site is altered because of local changes or changes in overall protein structure, the enzyme may be unable to bind to the substrate. For example, an enzyme can bind to a specific substrate at an active site. As we discussed earlier, a protein's shape is critical to its function.


 0 kommentar(er)
0 kommentar(er)
